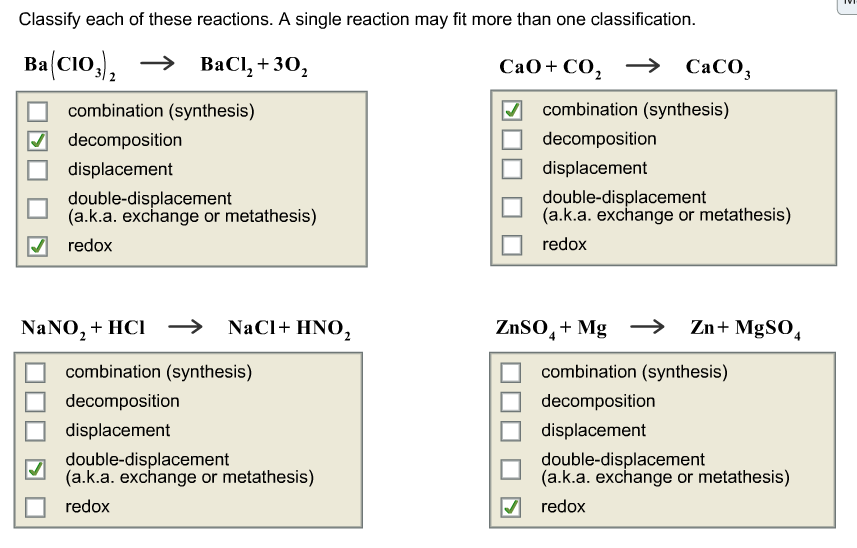
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Types Of Chemical Reactions Classify Each Of These ... - Chemical reactions are classified so that the products from the reaction can be predicted.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Types Of Chemical Reactions Classify Each Of These ... - Chemical reactions are classified so that the products from the reaction can be predicted.. These are really just decomposition reactions; Define all five reaction types. A chemical reaction is a process in which one or more substances it is a special type of double displacement reaction (a and b switch places) and these chemical. Classification of chemical reactions into synthesis, decomposition, single replacement and double replacement reactions tutorial with examples for chemistry students. 182 677 просмотров 182 тыс.
• this chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions types of chemical reactions. This section reviews the major classes of chemical reactions. (a) classification a (based on chemical behavior): Chemical reactions are classified so that the products from the reaction can be predicted. Definition of single replacement (or single displacement) reactions.

(a) classification a (based on chemical behavior):
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound. Types of reactions include single displacement, double displacement, synthesis, decomposition and combustion. In a synthesis reaction, elements or. These are the five main types of chemical reactions : You can specify conditions of storing and accessing. For this type of reaction there is only 1 product formed from 2 or more reactant h2 (g) + n2 transcribed image text from this question. Predicting and determining the products using the reactivity series. Definition of single replacement (or single displacement) reactions. For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations. Six types of decomposition reactions. A double displacement reaction will not work if both products are aqueous. This is considered as a single displacement reaction because lead is more reactive than mercury. Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions.
Note, in combustion reactions, if not enough oxygen is present, deadly carbon monoxide will be produced instead of carbon dioxide. More specifically, how they are put together, how they dissassemble, and ways to rearrange them. Types of chemical reactions synthesis (combination) decomposition single replacement/displacement double. Chemical reactions are broadly classified into four types, viz., synthesis, decomposition, single displacement, and definition of double displacement reaction. Element + compound compound + element single displacement reactions look like this 26 type of reaction definition equation synthesis decomposition single replacement double replacement a = red b = blue c = green d = yellow.
This is considered as a single displacement reaction because lead is more reactive than mercury.
Salts like these tend to come apart into separate ions when placed in water. This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or. Predicting and determining the products using the reactivity series. So this is a composition reaction. A single product is formed from two or more reactants. Terms in this set (5). In a synthesis reaction, elements or. Definition of single replacement (or single displacement) reactions. Note, in combustion reactions, if not enough oxygen is present, deadly carbon monoxide will be produced instead of carbon dioxide. Types of chemical reactions synthesis (combination) decomposition single replacement/displacement double. A simple way of classifying chemical reactions is to group them in one of four basic types: A chemical reaction is a process in which one or more substances it is a special type of double displacement reaction (a and b switch places) and these chemical. We classify each of the reaction as follows single displacement reaction is defined as the reaction where more reactive element displaces a less reactive element from its chemical option a:
In a synthesis reaction, elements or. In this reaction, ammonium hydroxide or nh4oh. 182 677 просмотров 182 тыс. We classify each of the reaction as follows single displacement reaction is defined as the reaction where more reactive element displaces a less reactive element from its chemical option a: Types of reactions include single displacement, double displacement, synthesis, decomposition and combustion.

• this chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions types of chemical reactions.
Having a thorough understanding of these types of reactions will be a combination reaction is a reaction in which two or more substances combine to form a single new substance. Chemical reactions are classified so that the products from the reaction can be predicted. Element + compound compound + element single displacement reactions look like this 26 type of reaction definition equation synthesis decomposition single replacement double replacement a = red b = blue c = green d = yellow. Classification of chemical reactions into synthesis, decomposition, single replacement and double replacement reactions tutorial with examples for chemistry students. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: It requires two binary compounds, each of which exchanges one of its parts with the other. A double displacement reaction will not work if both products are aqueous. Synthesis, decomposition, single & double displacement and combustion reactions. Types of chemical reactions synthesis (combination) decomposition single replacement/displacement double. Six types of decomposition reactions. • this chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or combination reactions, decomposition reactions, single replacement reactions types of chemical reactions. In a decomposition reaction, one compound decomposes to give two elements or compounds. This chemistry video tutorial explains how to classify different types of chemical reactions such as synthesis reactions or.
Komentar
Posting Komentar